Thailand FDA Medical Device Registration Overview
Thailand is one of the major medical device markets in South East Asia. Medical Devices in Thailand are regulated by the Thailand Food and Drug Administration (TFDA) under the Ministry of Public Health (MOPH), Thailand. Foreign companies must appoint a Thailand Local Authorized Representative for assisting them with the Thailand FDA Medical Device Registration process.
Regulatory Authority: Thai Food and Drug Administration (TFDA)
Regulation: Medical Device Act of 2008 (B.E. 2551) and updated by the Medical Device Act / Ordinance B.E. 2562 (2019) (Issue 2)
Authorized Representative: Thailand Local Authorized Representative
QMS Requirement: ISO 13485:2016
Assessment of Technical Data: Medical Device Control Division (MDCD)
Validity of License: 5 Years
Labeling Requirements: Section 44, Medical Device Act of 2008
Submission Format: e-submission
Language: Thai or English
For Home Use Medical Devices: Thai language is mandatory
In 2021, the Thai Food and Drug Administration has revised their medical device regulations. As per the older regulations, 90% of the devices were categorized as general devices, requiring validation and not a detailed TFDA review. In contrary, as per the revised regulations, which are aligned with the regional ASEAN Medical Device Directives (AMDD), majority of the devices are now under the TFDA’s scrutiny, requiring either notification or registration.
Thailand Medical Device Classification
As per the old regulations, medical devices were classified into three (3) Classes – General, Notified and Licensed medical devices corresponding to Class III, II and I respectively. The Class I devices were of high-risk and Class III were of low-risk. According to the new guidelines, the medical devices are classified into four (4) Classes – I, II, III and IV. Unlike the older regulations, Class I devices impose lower risk and Class IV devices impose higher risk to the end-users. The new regulations also include 3 rules on grouping, allowing the devices and IVDs to be grouped to Single, Family, System, Set, IVD test kits and IVD cluster. The devices shall have one generic proprietary name, product name and common intended purpose to be grouped into any of the above listed categories.
|
Class |
Risk |
|
Class I |
Low-risk |
|
Class II |
Low to Moderate-risk |
|
Class III |
Moderate to High-risk |
|
Class IV |
Thailand Local Authorized Representative
For foreign medical device companies that do not want to set up their own subsidiary or trust a local distributor with their product registration information, an independent third party acting as Thai legal agent is the best choice. Employing a Thai third party local agent to register medical products with the TFDA and to act as Thailand Legal Representative allows the manufacturer to easily change distributors.
Thai FDA Medical Device Registration
The registration requirements of the device vary with the class of device. The low-risk Class I devices must be listed before they are imported and marketed in Thailand, whereas the Class II and Class III devices have to be notified and the Class IV devices must have an approved license to place them in the Thai market. The Class II, III and IV devices require submission of technical dossier, as per ASEAN CSDT format. The Class I Sterile and measuring devices require submission of testing reports for placing these devices in the market.
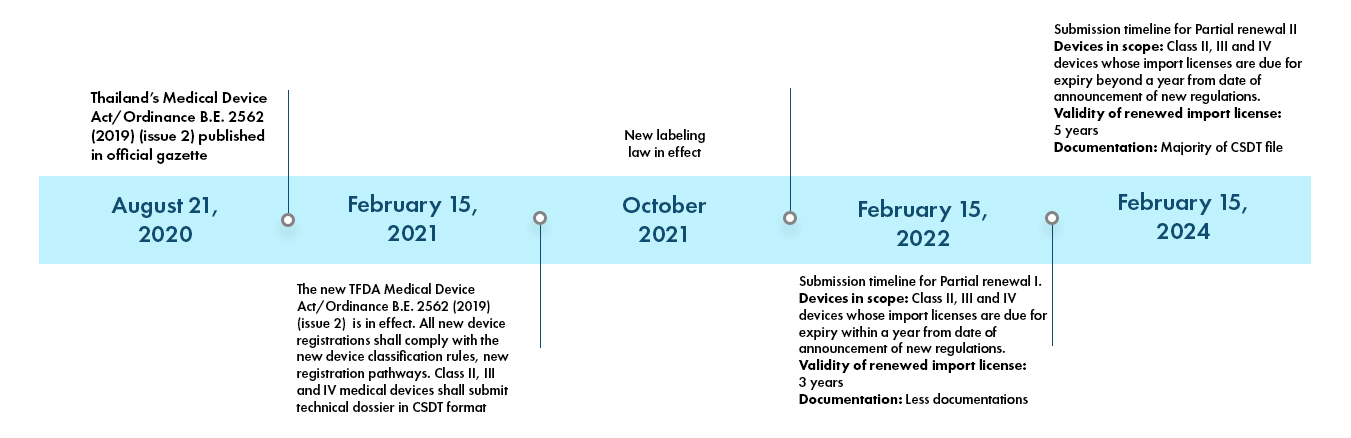
All devices registered after February 15, 2021 shall comply with the new regulations and shall have their technical files compiled in CSDT format. The devices currently approved under the old regulations shall be renewed as per new regulations. These devices, however, have a grace period based on the expiry of existing TFDA approvals. The Class II, III and IV devices with their certificates due for expiry within one (1) year from date of announcement shall renew by February 15, 2022. Referred to as partial I, this partial renewal requires less documentation and renewed import license would be valid for three (3) years. The class II, III and IV devices due for expiry after a year from date of announcement i.e., in 2022 and beyond, can submit the applications within three (3) years and requires majority of CSDT document to be submitted. Referred to as partial II, the import licenses renewed under this will be valid for five (5) years. Once, these renewed import licenses are expired, a full submission is required.
Thai FDA announced new guideline on 16th March 2022 on E-submission system. Once the License holder submits the required documentation in the E-submission system according to the guideline published by MDCD, order of payment will be generated, after receiving the payment, the Thai FDA will evaluate the documents.
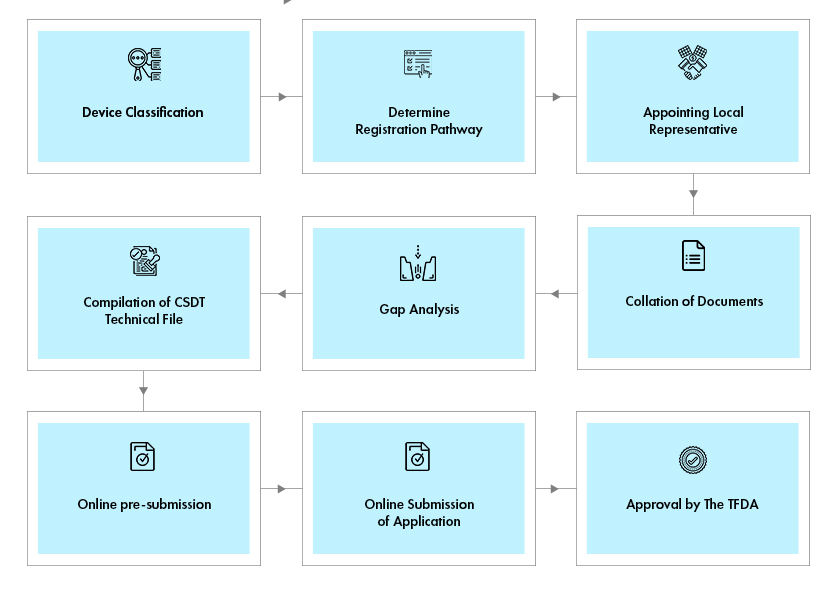
Process flow
Freyr supports foreign manufacturers in end-to-end Medical Device lifecycle management, including post approval activities, such as:
- Post approval change management - modifications to existing Medical Device approvals such as, addition of new variants, accessories, addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between the TFDA and the manufacturer
- Importation management
- Handling of complaints or adverse events and reporting to the Health Agency
Freyr as a leading Regulatory consultant can assist you through the Thai registration and licensing processes and secure approval for your Medical Device.
Summary
|
Device Class |
Risk |
Registration pathway (application type) |
TFDA Timelines |
Validity |
Total fee structure |
|
Class I |
Low-risk |
Listing (self-declaration) |
200 days |
5 years |
3,100 Bhat (US $105) |
|
Class II |
Low to Moderate-risk |
Notification (CSDT) |
250 days |
5 years |
31,000 Bhat (US $1,080) |
|
Class III |
Moderate to high-risk |
Notification (CSDT) |
250 days |
5 years |
31,000 Bhat (US $1,080) |
|
Class IV |
High-risk |
License (CSDT) |
300 days |
5 years |
51,000 Bhat (US $1,775) |
Freyr Expertise
- Regulatory due-diligence and Regulatory Intelligence (RI) services
- Device classification and grouping services
- Listing services for low-risk devices
- Notification services for moderate-risk devices
- Transition for new regulation compliances
- Licensing services for high-risk devices
- Legal representative services
- Labelling services
- Translation Services
- Distributor identification and qualification services
- Post-marketing surveillance services
- Post approval change management
- License renewal and transfer services
